The Pharmaceutical Industry has experienced a rapid expansion in the last few years. As a result, the need for strong compliance management becomes all the more indispensable. Performing regulatory scrutiny during every process of a pharmaceutical company, from start to finish is a long and exhaustive process.
Not only the process is time-consuming, but it is expensive too. The costs incurred in obtaining FDA approvals and the expenditure in producing consistently high-quality products are significantly large.
To overcome such drawbacks and keep pace with the increasingly pushing demand, one needs to deploy compliance management software in their respective pharmaceutical companies.
Pharma ERP- The Secret To Success
One reliable and end-to-end complete solution that can reduce compliance complexities is Enterprise Resource Planning (ERP) software. As the effective and efficient compliance capabilities of an ERP aid in crisis management and targets broader risk management programs.
With it, the companies can look holistically across the strategic, operational, and other production activities. It not only optimizes the decision-making but also mitigates risks. When the compliance is met within the entire supply chain, the result is a greater focus on operational flow and impactful marketing activities to boost sales.

A Look at the Figures-
Out of hundreds of drugs each year, only about 10% of drugs end up receiving FDA approval.
In 2019, only 59 drugs received approval, and in 2020, 53 made the cut.
Source- Pharmaapproach.com
How Does ERP Help Achieve Compliance Standards?
Even though the pharma industry is highly challenging, insights management can help manufacturers ensure better compliance management. This is where ERP comes in.
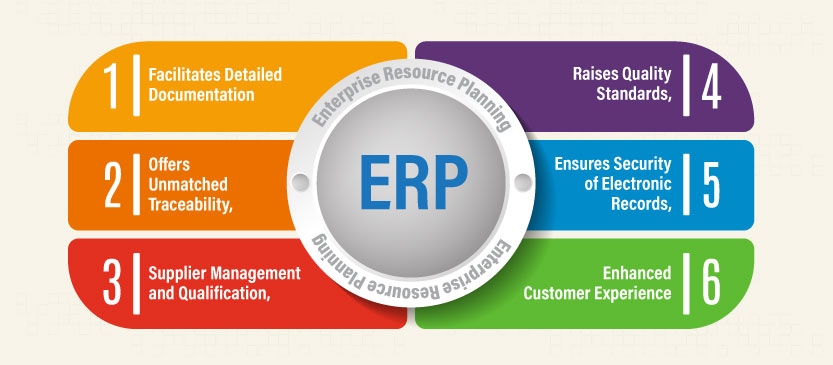
- Facilitates Detailed Documentation
Extensive, detailed documentation is crucial to the pharmaceutical industry. As it helps to establish transparency and resolve issues quickly. Documentation includes information about the regulations that companies must comply with, the details of systems installed, vendor’s details, QC specifications, reports, and inventory movement.
ERP is capable of generating this all alone in a systematic manner along with printing Batch Manufacturing Records, where-used reports, and product labels.
- Raises Quality Standards
The need for quality maintenance is critical in a compliance-regulated environment for pharmaceutical companies. ERP provides a comprehensive framework for managing product innovation with solid product data management, along with quality process controls. It performs multiple complex processes like:
1. Addressing quality issues more quickly, thus saving time and effort that contribute to the company’s bottom line.
2. Using the adverse Event reporting feature during Audits and as a reference in the future.
3. Performing Non-Conformance and Corrective and Preventive Actions (CAPA) in case of a failed QC test
Moreover, the execution of QC becomes a cakewalk with convenient sampling plans and stability testing. Batch monitoring activities and periodic Inspection plans under electronic execution eliminate chances of rework and rejections.
- Offers Unmatched Traceability
Having in place a clear, effective, and broadly communicated compliance program helps you signal to the auditors that compliance is the top priority
for your company. It shows your commitment to doing business the right way, and to the highest ethical standards.
When you can track and trace your products bi-directionally, the recall becomes easier than ever and the capability to take action faster.
By demonstrating your expertise in the field of all the relevant laws and regulations, your brand name creates a powerful impact in the market. Customers, employees, and vendors seeing this commitment gain a feeling of trust in you, and such trust breeds brand loyalty.
- Ensures Security of Electronic Records
Data security is crucial in manufacturing, whether it’s regarding formulas, material substitution, or sensitive reports and customer order details. One increasing concern is controlling breaches of data security.
ERP systems provide a complete set of security features including access controls, data encryption, and server-based processing.
- Data Encryption – ERP Security feature gives you visibility to transactional data
- Audit Trails – Keeps records about all the revisions with their date and time in the master database.
- Electronic Signatures -Secured transactions by an authorized operator maintain the security of transactions. It also creates a summary and detailed log of all transactions for audits.
- Supplier Management and Qualification
With procurement from a verified and certified source turned mandatory in the Pharma industry, ERP ensures purchasing of material from certified vendors and keeps records for the best buy in the future.
Along with maintaining vendor details like the discount price and special offers, ERP also prompts when their certification is about to expire, ensuring compliance with the rule.
- Enhanced Customer Experience
When quality, documentation, and traceability are taken care of well by ERP, the manufacturer can focus on other key factors of a business like a customer management.
Customer responsiveness is improved due to transparency in the system and the information is furnished in a blink of an eye. Pharma ERP also streamlines sample management and enables tracking of customer complaints in an efficient manner.
Conclusion
A good compliance program can have significant, positive, and noteworthy benefits on business operations. The advantages are many and vary with the nature of the business, but the above top six business benefits of compliance in the pharmaceutical industry are most prominent and cannot be overlooked.
Implementing ERP helps in handling compliance in the pharmaceutical industry, which opens doors for greater business opportunities within the country and outside too. It even keeps the warning letter, recall notices, and other unwanted situations, bringing about huge benefits in the long run.
So gear up for compliant manufacturing and operations with an ERP that’s built specifically for the Pharma industry.