ERP for Biotechnology
Major Challenges Faced by Biotechnology Manufacturing Industry?
The biotechnology segment in the global life sciences industry continues to grow at a rapid pace due to technological advancements and availability of low-cost biosimilar drugs. But this rapid growth has resulted in an increase in competition too. As a result, biotechnology manufacturers are pursuing ways to deliver these drugs at an affordable price without compromising on safety and quality.
In doing so, they face challenges such as stringent regulatory compliances and safety requirements, and need to shorten the product development cycle, among others.
To successfully navigate through such challenging market conditions, biotechnology companies need to empower themselves with an integrated ERP software for biotechnology. One, that precisely leads the product lifecycle from R&D to distribution including all the ever-changing regulatory requirements.
Would you like to learn more about our ERP for Biotechnology?
Food Industry
How does ERP help in Biotechnology Manufacturing Industry?
A solution that understands the complex needs of this industry is BatchMaster ERP for Biotechnology, tailor-made for this segment and its obligatory regulatory compliance needs which include adherence to cGMP requirements as well. BatchMaster ERP for pharmaceuticals is specifically designed to address the issues that pharma manufacturers face and automates the entire supply chain cycle which makes this software a perfect fit for biotechnological manufacturers too.
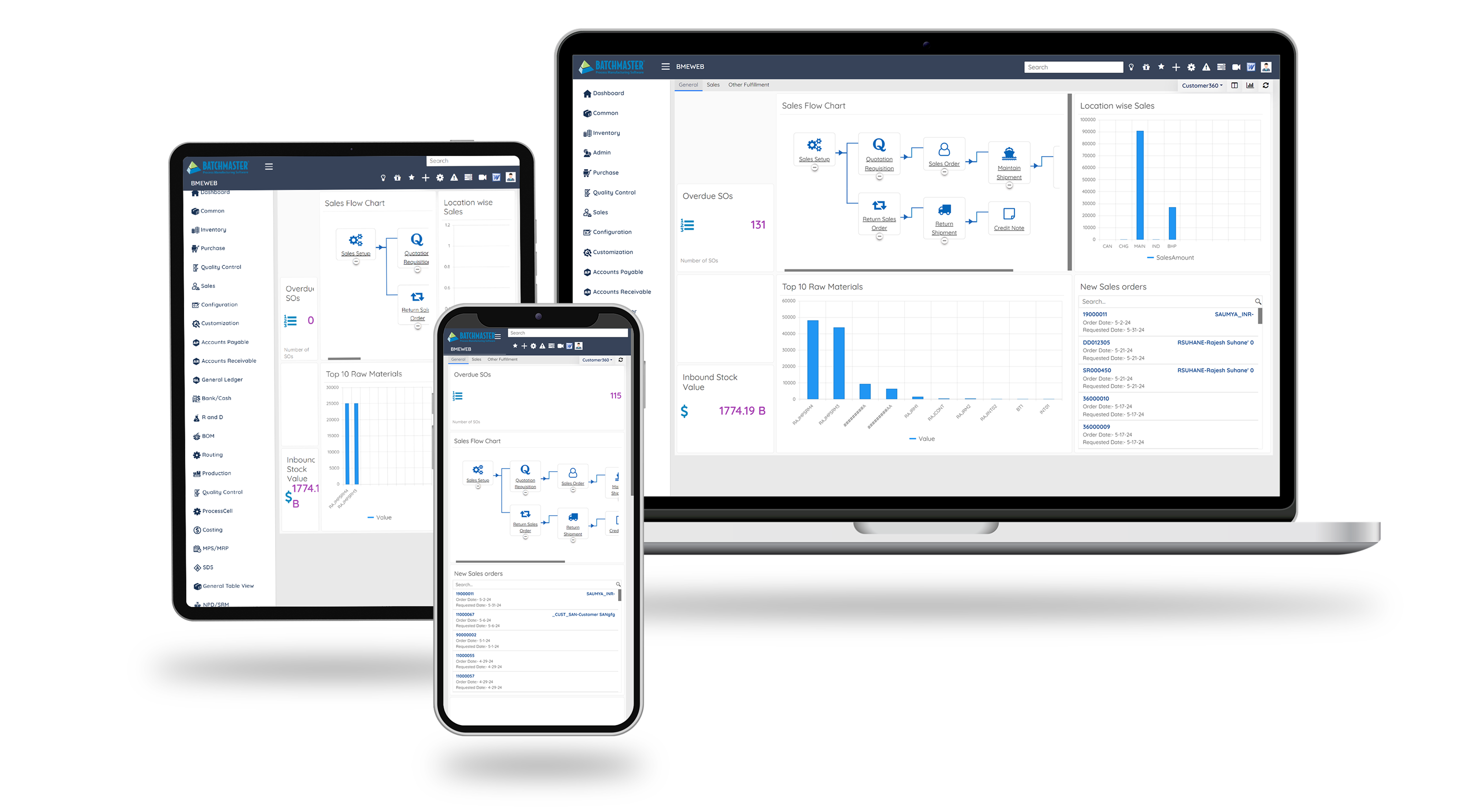
BatchMaster ERP for Biotechnology can very easily map biotech companies’ basic as well as complex business processes like formulation, batch management, supplier verification, generation of BMR etc., and provide real-time information of all the departments, which helps manufacturers to streamline their operations and take timely proactive informed decisions to outlast the competition.
Our products have a proven integration to SAP Business One, Sage 50/100/200/300, QuickBooks, and Tally.ERP 9 which means you can easily upgrade to BatchMaster without having to shun your financial software.
Transform Your Biotechnology Manufacturing Business with BatchMaster ERP
Unlock Predictive Insights, Drive Success, and Achieve Amazing Results!
Key Features of Biotechnology Manufacturing ERP Software
- Raw material planning and procurement
- Supplier/Vendor management and qualification
- FDA 21 CFR part 11 compliance, which includes secured master data management
- Quality Control (QC) with sampling plan, stability testing
- Quality Assurance (QA) as per cGMP norms
- Opportuity tracking, sales, delivery and invoicing
- Inventory management, including material characteristics such as potency, catch weight, classifications, expiration dates, lot control, and varying units of measure
- Support to contract manufacturing
- Batch monitoring activities by inspection plan
- Formulation management: Complete formula security with audit trail and approval workflow. Also includes by-products, co-products, intermediates, variations in expected yields, and alternate equipment-specific formula
- Electronic execution of SOPs by inspection checklist
- Electronic batch ticket, electronic weigh sheet, and manufacturing batch record (MBR)
- Non-conformance (NC)
- Corrective and preventive action (CAPA)
- Easy material substitutions
- Multiple packaging of same formulation
- Shelf life/Expiration support
- Bi-directional lot traceability and lot recall
- Separate granular costing of formula and packaging material
- 'What-if' analysis and various costing methods for increased margins
- Adverse event/Complaint reporting
- Allergen management
- Mobile warehousing: Employment of handheld RF devices to execute material movement and adjustments within one or more facilities
- Dedicated module to handle all complaints, returns and recalls
- Master Production Scheduling (MPS)
- Material Requirement Planning (MRP)

Benefits of BatchMaster ERP in Biotechnology Manufacturing
- Streamlined operational process flow and enhanced profits
- Business efficiency
- Cost control through reduced wastage, optimum material utilization, and inventory management
- Real-time batch monitoring to make accurate business decisions
- Greater customer satisfaction by swift response to customer demands
- Assured quality and timely supply
- Competitive advantage
- Support for cGMP, and other stringent compliance regulations
- Expanded approval controls
- Enhanced speed to introduce drugs to market that are cheaper than the competitors
- Ability to maintain formula secrecy
Brochures
Run Our Manufacturing ERP With Your Existing Financials
Upgrade to our ERP without missing a beat in your financial and accounting routines. BatchMaster ERP offers seamless integration with Tally, QuickBooks, Sage 50/100/200/300, SAP Business One and other popular systems, ensuring a smooth transition.
Web/Cloud Deployment Available
Give your small, mid-size, or large-scale businesses the power of our ‘Cloud ERP’ solutions, and enjoy the benefits of leveraging the cloud.
Looking To Find Best Solution for Your Business?
Allow our expert team of solution consultants to review your business operations so that they can offer you the best-possible solution, either on premise or in the cloud, to meet all your industry-specific needs























