If you ask what the single most important facet of a product is, quality is the answer you are likely to get 10 out of 10 times; irrespective of what the product type is.
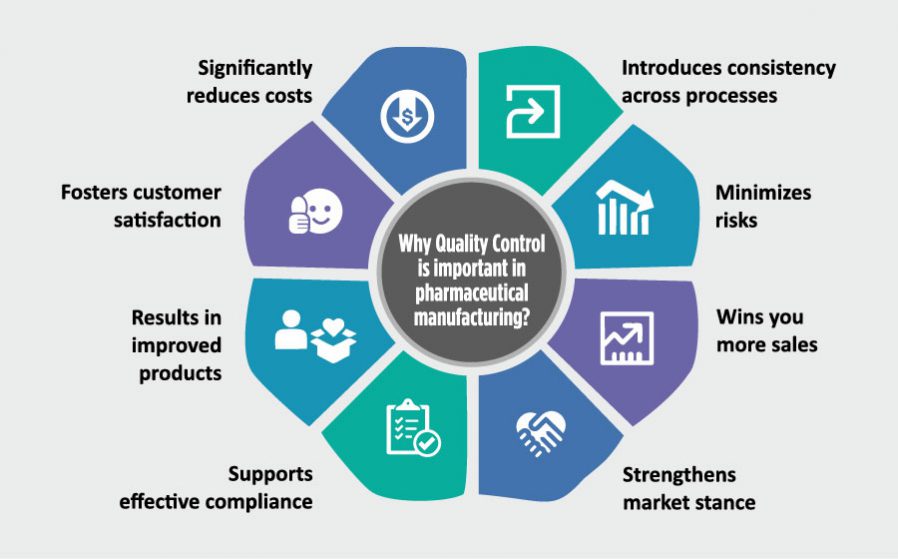
Such is the significance of quality that while its presence builds trust and drives sales, the lack of it can result in losing customer for good.
It is actually difficult to find people who are willing to compromise on the quality for any other benefit, and easy to find those who feel it is an indispensable and integral part of any product.
And hence, it is only fair to say that quality is the most crucial aspect any business must focus on to ensure its success.
When it comes to process manufacturing industry — pharmaceutical manufacturing in particular — the significance of quality goes several notches higher.
Quality becomes all the more paramount and non-negotiable for this industry, which happens to be one of world’s most highly-regulated sectors.
The products in this industry go through several rounds of scrutiny, as even the slightest of deviation in their quality could prove to be the difference between life & death- both literally and figuratively.
That’s why the pharmaceuticals manufacturers strive to ensure quality at every level of manufacturing, and develop effective and reliable products that meet the strictest quality standards.
And to do so, the pharmaceuticals manufacturers need a tool that can assist them in producing cost-effective yet quality drugs, and help control quality throughout the production process.
Modern-day pharmaceutical quality control software such as the enterprise resource planning (ERP) systems have emerged as that tool for them, as their presence can help the manufacturers ensure the products meet the requisite safety and quality requirements.
At the same time, the absence of a pharma ERP software, which comes loaded with the Quality Control (QC) functionality, may not just increase the risk of poor quality, but also result in higher cases of pharma product recalls, among other things.
By helping control quality in pharmaceuticals manufacturing, an ERP can make the life less stressful for the pharma manufacturers by allowing them to maintain the quality & safety standards of their products, besides facilitating better decision-making.
How exactly can quality be controlled in pharma manufacturing, and what role does a pharma quality control software play in it, we shall read in this blog.
Quality – An Inherent Part of Process Manufacturing Ecosystem
In the pharmaceutical manufacturing industry, it is essential that every single drug/medical device that reaches the end consumer should be fit for consumption.
Thus, it is important that it strictly adheres to the quality and safety standards.
For that the manufacturers need to ensure Quality Control is an integral part of their organization’s quality system – other parts of which are compliance, audit, cGMP, etc.
The Quality system enables the manufacturers to manufacture products that are pure, safe, effective and reliable.
The evaluation of safety and efficacy and their maintenance in practice is dependent upon how quality is controlled in pharmaceutical manufacturing.
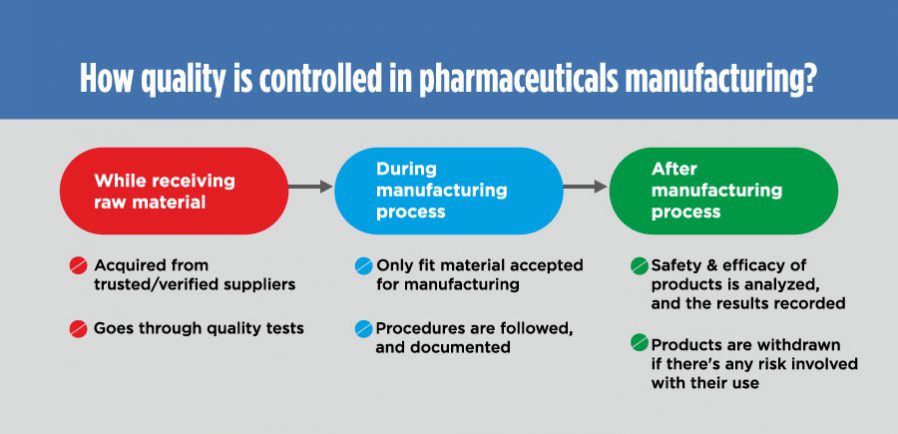
Usually, it is controlled at every level; broadly at the time of:
Receiving raw material:
The raw material is only bought from the FDA-approved and verified suppliers. As a standard operating procedure (SOP), any raw material (active ingredients, excipient, packaging materials, etc.) that enters the four walls of a pharmaceutical manufacturing unit is placed in quarantine area.
There are inspection plans and checklists in place, which help identify which raw material is fit and which one needs to be rejected.
The raw material is accepted for further processing only after clearing all the requisite tests for quality standards and specifications.
During manufacturing process:
Once accepted, the raw material proceeds towards manufacturing section.
Here, each process needs to be properly documented so as to establish evidence that it was executed within the pre-defined quality parameters, according to cGMP.
Every manufacturing unit has few documents that help them to maintain and sustain quality standards.
MBR (Master Batch Record) and BMR (Batch Manufacturing Record) are two such documents.
MBR are general manufacturing instructions that contain all pharmaceutically relevant data such as the input material list, valid SOPs, detailed work instructions to be applied in production, etc.
BMR, on the other hand, is a written document that contains step-by-step process for manufacturing each batch; like when, how, by whom, with what tools, and in what environment a product was produced.
It also carries data such as the batch records, Bill of Materials, equipment cleaning record, etc., and acts as a proof that batches were properly made, and checked by quality control personnel, and each processing step was completed according to the MBR; thus ensuring quality.
After manufacturing:
Samples are taken and tests are conducted on them for quality and specification standards after the manufacturing process.
Distributors and the distributed products are tracked as virtually every aspect is serialized, so that the offending products can be withdrawn from the market if required.
Strict guidelines are followed during pharmaceutical manufacturing.
The procedures are followed, and documented, products are analyzed, and results are recorded.
The safety, efficacy and stability of the products is measured through different means, and they are released to the market only if they are fit.
If any unexpected result comes out post their release, the results are monitored, recorded, and reviewed. The products are withdrawn from the market if there’s any risk involved with their consumption.

ERP software plays its part well
An efficient pharma ERP helps the pharma manufacturers maintain safety & quality of pharmaceutical products in many ways.
Using such ERP software, the pharma manufacturers can define inspection plans and inspection checklists.
While the former allows maintaining information on how an item (finished good, raw material, equipment etc.) is to be inspected, how the inspection is to take place, the item characteristics to be inspected and all the required test equipment that are needed for the inspection, the latter allows verification of whether or not a particular item meets the desired specification as mentioned against its name on the checklist during verification.
Accordingly, it can be determined whether the item is within tolerance or should be rejected if it doesn’t conform to the defined standards.
And so, only the quality items that meet the set standards are used for production.
What a good ERP software for pharmaceuticals also does is that it allows creation and storage of QC tests with predefined parameters for particular items/item group through its QC module.
Manufacturers can manage the records of passed/failed tests, accept/reject the product based on inspection result, and initiate actions like swift recall, destruction etc. against the items that fail inspection.
This way, they can ensure quality at all stages of production.
By making the QC test results available to the dashboard & reports, ERP software equips pharma manufacturers with in-depth information of the entire test case history.
The manufacturers have the information on how many times the test has been executed, the performance of the test case, its status, etc.
This way, they can make informed decisions with respect to the quality of the product by easily analyzing the QC results.
Lastly, an ERP for pharmaceuticals manufacturing ensures that the events of non-conformance (NC) are identified on an immediate basis in order to take corrective action for the same and minimize the damage/loss.
An ERP software for pharmaceuticals with NC & Corrective Action Preventive Action (CAPA) functionality quickly identifies and responds to any current or potential product quality risks, and failures or customer complaints, thus ensuring overall product quality.
Besides, an ERP software for pharmaceutical quality control also helps meet the cGMP as well as all the regulatory compliance requirements with a dedicated module for the same, and thus keeps a pharma manufacturing business audit-ready.
Conclusion
Quality control is an extremely significant part of any pharma manufacturing company’s quality system. And an ERP software facilitates controlling quality throughout production and meeting the quality & safety requirements.
With its QC capabilities, the pharmaceutical quality control software supports QA testing, documenting the entire process and the outcome of each test, and eliminating any slight/major slip-ups, to ensure conformance with expected results.
This means only the materials fit for consumption are sent for approvals while the inappropriate ones are rejected and sent for quarantine.
Moreover, its compliance module allows meeting the cGMP as well as all other regulatory requirements.
If you’re looking for a pharmaceutical quality control ERP system for your pharma manufacturing business, visit our website today.
You’ll get detailed information about us and our various offerings. You can also get in touch with our experts to schedule a demo.